Wearable devices offer new opportunities and new challenges for drug discovery
Katy Scott | July 7, 2022
Not just Watches
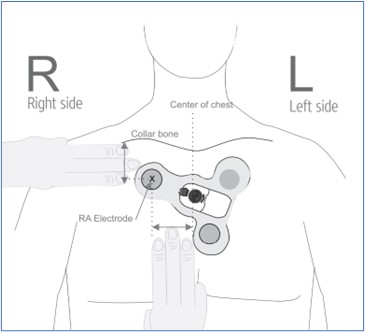
Including devices besides smart watches, health and wellness related wearables are expected to continue to improve as a technology and increase market growth in coming years (Loucks, Bucaille, Stewart, & Crossnan, 2021). The Food and Drug Administration issued Emergency Use Authorizations for 6 wearable monitoring devices to protect health care worker safety against COVID in 2019 (Food and Drug Administration, 2021). This is a placement diagram for one of those devices, a Vital Signs Monitoring System, which tracks electrocardiogram data and downloads it to a smartphone:
Emerging Opportunities
With increasing ubiquity and accuracy of wearable health monitors, it follows that investigators are exploring options to leverage them to improve clinical trials management. Between 35% and 65% of the cost of a clinical trial goes to site focused costs (Serkaya, Wong, Jessup, & Beleche, 2016). A shift toward remote administration using automated data collection and delivery through wearable devices could drive substantial savings. Double digit percentages in development costs could have disruptive effects in the health care industry, making more therapies available to patients at costs they can afford.
In addition to cost savings, at-home biometrics monitoring may overcome barriers to access. For example, some patients decline or attrit from clinical trials due to costs or inconvenience of traveling to a trial site (Thoma, Farrokhyar, McKnight, & Bandari, 2010). As well, some underserved communities enroll less often in trials due to mistrust of the medical experiments (Institute of Medicine (US), 2012); they may feel more comfortable participating from home. Furthermore, some patients, like those suffering from a severe subgroup of Myalgic Encephalomyelitis, cannot make office visits without suffering harm (Centers for Disease Control and Prevention, 2019). At-home monitoring could make study of their disease and progress toward treatments possible.
Unique Challenges
The use of wearables data for clinical trials experiments presents unique challenges compared to a traditional clinical trial. Data integrity and security must be ensured throughout data storage, transmission, and retention. If the data pipeline relies on personally owned Wi-Fi networks and smartphones or tablets, a variety of security solutions would need to be established and maintained throughout trial operation. Reliance on personal infrastructure for data transmission could introduce bias against individuals or communities with less access to internet connectivity or smart devices.
A wearables experiment may need to be designed differently compared to a traditional trial. For example, individual sensors would be used continuously per patient, amplifying potential measurement bias. As well, patients may have more visibility to regular biometrics monitoring which could introduce placebo effects. Finally, the experiments may see greater attrition in subjects due to loss of devices. With measurement assets not under control of the clinic, they risk or damage, theft, or loss before completion of the trial. Investigators would need to plan for all these effects in their experimental design.
A Smart Future
The healthcare industry is ready for the cost savings and equity in recruitment these devices can offer. As long as data security and experimental designs keep pace with the development of wearables, these fun and fashionable accessories could revolutionize medicine in coming years.

References
Centers for Disease Control and Prevention. (2019, November 19). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome- Severely Affected Patients. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/me-cfs/healthcare-providers/clinical-care-patients-mecfs/severely-affected-patients.html
Food and Drug Administration. (2021, July 15). Remote or Wearable Patient Monitoring Devices EUAs. Retrieved from U.S. Food and Drug Administration: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/remote-or-wearable-patient-monitoring-devices-euas
Institute of Medicine (US). (2012). Public Engagement and Clinical Trials: New Models and Disruptive Technologies: Workshop Summary. (p. Working with Underserved Communities). Washington DC: National Academies Press (US).
Loucks, J., Bucaille, A., Stewart, D., & Crossnan, G. (2021, December 1).
Wearable technology in health care: Getting better all the time. Retrieved from Deloitte Insights: https://www2.deloitte.com/us/en/insights/industry/technology/technology-media-and-telecom-predictions/2022/wearable-technology-healthcare.html
Serkaya, A., Wong, H.-H., Jessup, A., & Beleche, T. (2016, April). Key cost drivers of pharmaceutical clinical trials in the United States. Retrieved from NIH National Library of Medicine: https://pubmed.ncbi.nlm.nih.gov/26908540/
Thoma, A., Farrokhyar, F., McKnight, L., & Bandari, M. (2010, June ). How to optimize patient recruitment. Retrieved from NIH National Library of Medicine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2878987/
